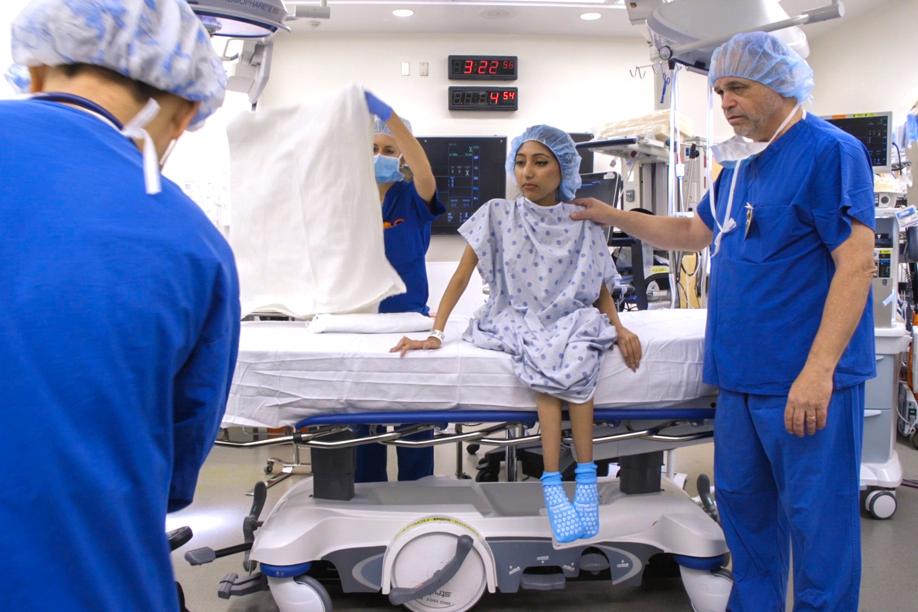
BALTIMORE — Dr. Thomas Crawford held up a tiny glass vial while an anesthesiologist inserted a hypodermic needle into the narrow opening, carefully drawing out a single dose of one of the world’s most expensive drugs.
“Five mils of this stuff is $125,000,’’ the neurologist said, sounding incredulous.
Under Crawford’s watchful eye in a Johns Hopkins Hospital surgical room, the anesthesiologist slowly injected five milliliters of the clear liquid — a drug called Spinraza — into the back of Farihah Mehmud, a 20-year-old college student.
Mehmud, who had received a local anesthetic, sat on the edge of a gurney, showing little discomfort as the medicine flowed into her spinal canal. She hopes the treatment — sold by Biogen Inc. of Cambridge — will halt the relentless progress of spinal muscular atrophy, the inherited neuromuscular disease that affects about one in 10,000 babies born each year. She has had to use a wheelchair since kindergarten.
Brynne Willis, a 25-year-old treatment coordinator, chatted with Mehmud. Willis also has SMA, albeit a milder form. At 10 years old, she was diagnosed by Crawford. He hired her in 2015, thinking she would have a rapport with patients. Willis, unlike Mehmud, can walk but tires easily. She hopes to be treated with Spinraza, but has put it off because of her work schedule and ongoing graduate studies.
“I told the physicians here that I’m on the back burner — get to me when you can,’’ Willis said.
At Johns Hopkins and other hospitals around the country, the procedure itself is the easy part of the Spinraza story. The hard part is getting insurers to cover the cost: $750,000 in the first year and $375,000 annually afterward.
Mehmud’s insurance plan, the state-run Medicaid program in Maryland, will only pay for the cost of administering Spinraza, not the drug itself. After receiving two denials for coverage, she contacted Biogen. The company agreed to provide Spinraza free in the first year, but after that it’s unclear what will happen.
Government and commercial insurers across the United States generally have been more willing to cover infants with the deadliest form of the disease, typically diagnosed within the first six months. Sometimes described as “Baby ALS,’’ it is the most common genetic cause of death in infants. It accounts for nearly two-thirds of SMA patients. Most die by the age of 2.
Remarkably, in clinical trials, Spinraza not only halted the progression of that form of SMA but allowed patients to achieve physical milestones once considered unthinkable, including sitting, standing and walking.
For teenagers and adults whose SMA only became apparent after they were 6 months old, the benefits of Spinraza aren’t as obvious, which is why insurance companies haven’t been eager to pay for it.
These patients often have lost the ability to walk or are starting to have difficulty walking. Some have a hard time using their arms. Many develop curvature of the spine, requiring fusion surgery to straighten their backs and improve breathing, as Mehmud did when she was 12. That can make the spinal injections of Spinraza harder to give.
Mehmud, who needs assistance with routine activities such as getting out of bed and bathing, hasn’t felt any difference since starting the injections, but that’s not unexpected. She and her doctor hope the drug allows her to continue to manage simple but meaningful everyday activities, like brushing her hair.
“Just maintaining even what I have would be amazing,’’ said Mehmud, who attends Howard Community College in Columbia, Md., and lives in nearby Ellicott City, Md. “I cannot let life slip away.’’
Crawford, 63, has cared for hundreds of SMA patients during his career, going back to his days as a medical student in Los Angeles when he once stayed up through the night with an infant who was dying of the disease.
“In the morning, the attending physician came around and said, ‘SMA is such an awful disease. There’s nothing you can do — next case,’ ’’ he recalled. “This is one disease everyone was running away from because people said there’s no hope.’’
After Spinraza worked well in late-stage clinical trials last year, Crawford did a back-of-the-envelope calculation with several colleagues. He knew the drug would be expensive if the Food and Drug Administration approved it. He and his colleagues estimated that Biogen would charge about $250,000 for the first year.
After the FDA signed off on Spinraza on Dec. 23, Biogen priced it at three times that much.
“I was sort of appalled,’’ Crawford said. But at the same time, he was “mindful of the fact that this was unbelievably expensive’’ to develop.
Jonathan Saltzman can be reached at jonathan.saltzman@globe.com.



